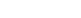
The all sliming cyanidation method is a common method for extracting gold from low-grade gold ores. During the production process, there are many factors that affect the efficiency and success of the all sliming cyanidation process, such as ore composition, ore particle size distribution, cyanidation agent concentration, cyanidation agent pH value, and stirring and oxygen supply during the cyanidation process, cyanidation time, temperature, etc. The following will introduce you to five key factors that affect the all sliming cyanidation of low-grade gold ore to help you better optimize and improve the gold ore cyanidation process.

(Gold ore)
Use the table of contents below to navigate through the guide:
01Composition of low-grade gold ore
The mineralogical characteristics of gold ore can significantly affect the cyanidation process. If low-grade gold ore is rich in sulfides (such as pyrite, yellow nickel, etc.), these sulfides are easy to react with the cyanide agent, consuming the cyanide agent and reducing the gold extraction efficiency. Sulfides can also produce toxic by-products that can harm the environment. Some low-grade gold ores contain oxidized gold minerals (such as iron oxide ore), which are relatively easily dissolved by cyaniding agents. Therefore, higher oxide content can facilitate gold extraction.
The particle size and distribution of gold in the ore also affects the efficiency of all sliming cyanidation. Smaller gold particles are generally more susceptible to dissolution by cyaniding agents, so appropriate ore crushing and grinding processes are required to ensure exposure of the gold particles.
02Low-grade gold ore particle size
In all sliming cyanidation, the ore usually needs to be finely ground to expose the gold particles for efficient cyanide leaching. The smaller particle size provides a larger surface area for more interaction between the cyaniding agent and the gold, helping to increase the contact between the cyaniding agent and the gold, thereby increasing the dissolution rate and extraction efficiency of the gold. Because the particles are smaller, cyanide can more easily penetrate into the particles and be dissolved by the cyaniding agent faster, thereby shortening the cyanide time. In addition, small particles are more easily suspended and mixed in the slurry, which helps ensure that the cyanide agent is evenly distributed throughout the slurry and promotes gold dissolution.

(Cyanidation process)
03Cyanide agent concentration
Cyaniding agent concentration has a significant impact on the efficiency and success of the whole-sludge cyanidation process for low-grade gold ores. Higher concentrations of cyaniding agents generally result in faster dissolution of gold. This is because the higher the concentration of cyaniding agent, the more rapidly the cyanidation reaction occurs on the surface of the gold particles. This shortens the cyanidation time and increases the gold release rate. In production, as the cyanide concentration increases, the gold recovery rate will also increase. However, higher concentrations of cyaniding agent generally increase production costs, so there is a trade-off between using enough cyaniding agent to increase extraction rates while avoiding wastage of cyaniding agent to reduce costs. The appropriate cyanidating agent concentration depends on several factors including ore characteristics, treatment objectives, cost constraints and environmental requirements. Beneficiation trials are often required to determine the appropriate cyanide concentration for a particular situation.
04pH value of cyanide liquid
Controlling the pH of cyanide liquids is critical. The pH level directly affects the activity of the cyaniding agent. Typically, the pH range for all sliming cyanidation is between 10 and 11. Within this range, the cyanide agent has better performance and can effectively dissolve gold. Deviation from this pH range, especially in the alkaline direction, may result in a decrease in the efficiency of the cyanidation agent, thereby affecting the extraction of gold. The pH level also affects the behavior of other alkaline and acidic substances in the ore. For example, ores with high carbonate content may cause an increase in pH, affecting the performance of the cyaniding agent. Therefore, additional measures in pH level control are required to adapt to the characteristics of different ores. The interaction of the cyaniding agent with the slurry is also affected by pH. Proper pH control helps ensure that the cyanide solution is fully mixed with the ore, greatly improving the gold extraction efficiency.

(Cyanidation process)
05Stirring and oxygen supply
Adequate mixing of the slurry and supply of oxygen are critical to the success of cyanidation. Stirring ensures constant contact between the cyanide liquid and the ore particles, promoting dissolution of the gold. Stirring can prevent the sedimentation or aggregation of ore particles at the bottom of the tank, ensure that the cyanide agent can evenly cover the surface of the ore, and avoid local cyanide deficiency. Oxygen supply is key for the cyaniding agent to oxidize gold and form soluble gold cyanide. Oxygen is an essential substance for oxidizing gold during the cyanidation process. The lack of oxygen will lead to a decrease in the gold extraction rate. The oxygen concentration in the cyanide solution needs to be maintained at a high enough level to ensure effective oxidation of gold. This can be achieved through mechanical stirring or a gas supply system. Temperature should also be considered during the cyanidation process, as temperature has a significant impact on the solubility of oxygen in solution. Generally, lower temperatures increase the solubility of oxygen, but ensure that the temperature of the cyanide solution does not reduce the cyanide reaction rate.

The above are the five factors that affect the cyanidation process of low-grade gold ores. In actual production, there are other factors that affect the cyanidation of all sliming, such as temperature, the presence of organic matter, and the ratio of total cyaniding agent to ore. Therefore, these factors need to be carefully optimized to improve gold recovery while ensuring that environmental and safety considerations are met to achieve efficient and green production.


 marketing@ytxinhai.com
marketing@ytxinhai.com  0086 13810327080
0086 13810327080 
































































































 CHAT
CHAT MESSAGE
MESSAGE