Nickel, a critical metal powering industries from electronics to renewable energy, is increasingly sourced from low-grade laterite ores as high-grade sulfide deposits deplete. For mining professionals and industry enthusiasts, the challenge of efficiently extracting nickel from these complex ores has long been a focus of innovation. Enter atmospheric pressure leaching (AL)—a cost-effective, energy-efficient alternative to traditional high-pressure acid leaching (HPAL) that’s transforming nickel recovery. In this article, we break down the science, benefits, and breakthroughs of atmospheric leaching for low-grade laterite ores, backed by cutting-edge research.
Use the table of contents below to navigate through the guide:
01Why Laterite Ores Matter (and Why They’re Challenging)
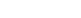
Laterite ores account for over 70% of global nickel reserves, yet their low nickel content (often 0.5-1.5%) and complex mineral structure make extraction tricky. Unlike sulfide ores, which are relatively easy to process, laterites are rich in iron (hydr)oxides like goethite [FeO(OH)] and hematite (Fe₂O₃), with nickel uniformly trapped within these minerals. Traditional HPAL systems require extreme temperatures (245–270°C) and pressures (4–5 MPa), demanding expensive specialized equipment and high operational costs. For mid-sized mining operations, HPAL is often prohibitively costly—creating a gap that atmospheric leaching aims to fill.

02The Basics of Atmospheric Pressure Leaching
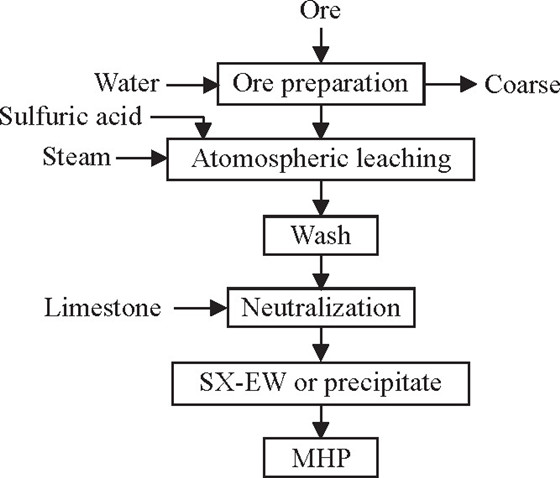
Atmospheric leaching operates at ambient pressure and moderate temperatures (typically 80–90°C), using acid as a lixiviant to dissolve nickel from ore. The process eliminates the need for high-pressure vessels, slashing capital expenditure and energy use. Key to its success is optimizing four critical parameters: lixiviant type, acid concentration, solid-to-liquid (S/L) ratio, and temperature.

Research on Iranian Sarbisheh laterite ore—characterized by 62% iron content and 0.8% nickel content—proves the method’s viability. The study found sulfuric acid (H₂SO₄) to be far more effective than hydrochloric acid, as it dissolves nickel while minimizing unwanted byproducts. Under optimized conditions (5 M H₂SO₄, 25% w/v S/L ratio, 90°C, and 2 hours of leaching), raw ore achieved a nickel recovery rate of 69%. But the real breakthrough came with pre-calcination.
Source: Published research on atmospheric pressure leaching of low-grade nickel laterite ores (Periodica Polytechnica Mining and Metallurgy).
03Pre-Calcination: Boosting Recovery to 95%
To overcome the limitations of leaching raw laterites, pre-calcination (heating the ore before leaching) emerged as a game-changer. Heating laterite ore converts goethite—a nickel-trapping mineral—into hematite (Fe₂O₃), which is far easier to dissolve. The Sarbisheh study tested calcination temperatures from 180–540°C and durations from 30–120 minutes, with striking results: at 540°C for 120 minutes, nickel recovery soared to 95%.
Thermal analysis (TGA/DTA) revealed why: goethite undergoes dehydroxylation at 280°C, breaking down its structure to release trapped nickel. XRD tests confirmed the transformation—after calcination, goethite peaks disappeared, replaced by hematite. This simple pre-treatment not only boosts recovery but also reduces acid consumption, addressing a major challenge of atmospheric leaching.
04Kinetics: Understanding How Nickel Dissolves
For industrial scaling, understanding leaching kinetics is crucial. A study used Shrinking Core (SC) models to analyze reaction rates, finding that nickel dissolution is controlled by chemical reactions at the ore particle surface—not diffusion. This was confirmed by an activation energy of 46.9 kJ/mol (well above the 40 kJ/mol threshold for chemical control). What does this mean for operations? Higher temperatures accelerate leaching: at 90°C, 54% of nickel was recovered in just 30 minutes, compared to 43% after 3 hours at 40°C. This allows for shorter batch times and higher throughput.
Click to view detailed information on laterite nickel ore processing technology.
05Why Atmospheric Leaching Beats HPAL for Low-Grade Ores
While HPAL remains effective for high-grade laterites, atmospheric leaching offers unbeatable advantages for low-grade deposits:
Lower Costs: No high-pressure equipment or extreme energy requirements, cutting capital expenditure by 30–40%.
Flexibility: Works for both limonitic and saprolitic laterites, adapting to varying ore compositions.
Sustainability: Reduced acid consumption (thanks to pre-calcination) and lower carbon emissions compared to HPAL.
Scalability: Ideal for small-to-medium mines, as it requires less infrastructure and operational expertise.

Xinhai’s Practical Experience in Laterite Nickel Processing
At Xinhai, we understand that laboratory results must be translated into stable industrial operation. Based on our experience in global hydrometallurgical projects, we provide:
Laterite ore mineralogical analysis and metallurgical testing
Customized atmospheric leaching flow sheet design
Calcination and leaching equipment selection
Acid balance and reagent consumption optimization
EPC+M+O project execution for nickel processing plants
Our engineering approach focuses on process stability, operating cost control, and long-term project sustainability, rather than theoretical recovery alone. If you are evaluating a low-grade laterite nickel project, Xinhai can support you from ore testing to full-scale plant implementation.


 marketing@ytxinhai.com
marketing@ytxinhai.com  0086 13810327080
0086 13810327080 
































































































 CHAT
CHAT MESSAGE
MESSAGE