Laterite ore processing refers to a complex metallurgical process specifically developed for extracting nickel and cobalt from laterite ore. Laterite ore is a material formed by surface weathering and is characterized by high iron content and low sulfide content.
Unlike sulfide ores, nickel laterite ore requires specialized nickel extraction processes due to its oxidized nature. With nickel prices fluctuating and environmental regulations tightening, choosing the right nickel laterite ore processing method can make or break a project's profitability. If you're dealing with challenges like low recovery rates or high energy costs in your operations, understanding these processes is key. Let's dive into the details to see how you can optimize your nickel ore processing operations.
Use the table of contents below to navigate through the guide:
01Understanding Nickel Laterite Composition: The Foundation of Effective Processing
Before choosing a nickel extraction process, you need to know what you’re working with—nickel laterite composition varies dramatically, and this dictates the best approach. These surface-weathered ores fall into two main types, each with distinct characteristics:
Limonite (Red/Yellow Laterite): High in iron (40–50%) and low in magnesium oxide (0.5–5%), with nickel content ranging from 0.8–1.5% and cobalt (a valuable byproduct) at 0.1–0.2%. Its chemical structure makes it ideal for hydrometallurgical processes, as high temperatures don’t play to its strengths.
Saprolite (Garnierite): Rich in magnesium oxide (15–35%) and lower in iron (10–25%), with higher nickel grades (1.8–3%) but minimal cobalt. This ore is amenable to pyrometallurgical methods, in which heat efficiently separates nickel from silicate minerals.
A third “transition zone” ore exists with medium nickel and iron levels, but no single proven process yet handles it economically.

02Overview of the Nickel Extraction Process for Laterite Ores
Nickel extraction process flows for laterite ores follow a general journey, starting from open-pit mining and ore preparation, then moving into beneficiation, metallurgical treatment, and final refining. Unlike sulfide ores, laterites rarely benefit from conventional flotation alone. Instead, success depends on chemical and thermal treatment.

After mining, laterite ores are usually crushed, screened, and sometimes washed to remove clay and fine gangue. From there, the ore enters either a thermal or hydrometallurgical route. Each route has its own logic, costs, and performance characteristics, which must align with the ore’s mineralogy and project goals.
☞Click to learn about the atmospheric leaching process for low-grade nickel laterite ore.
03Key Nickel Extraction Processes for Laterite Ores
When it comes to nickel laterite ore processing, two technologies dominate: pyrometallurgy (high-temperature smelting) and hydrometallurgy (chemical leaching). Hydrometallurgy employs chemical solutions or solids (such as acids) as reducing agents for leaching. Alkaline leaching (ammonia leaching) is the optimal method yielding the highest nickel recovery rates. Pyrometallurgy, however, requires high temperatures up to 1800°C, resulting in high energy consumption and significant carbon consumption. Detailed descriptions of these two methods follow.
1. Pyrometallurgy: High-Temperature Solutions for Saprolite Ore
Pyrometallurgy is the go-to for saprolite, leveraging heat to reduce nickel oxides into usable alloys like ferronickel or nickel pig iron (NPI). The most widely used method here is the Rotary Kiln-Electric Furnace (RKEF) process—a mature, reliable technique.
The RKEF workflow is straightforward: first, the ore is dried and pre-reduced in a rotary kiln to remove moisture and prepare nickel oxides for smelting. Next, it moves to an electric furnace where, with the help of reductants like coal, nickel and iron merge into ferronickel (Ni content 15–25%).
For clients seeking lower upfront costs, the blast furnace process is another option, using coke as a reductant to produce low-grade ferronickel. However, RKEF offers higher nickel recovery (92–97% vs. 80% for blast furnaces) and better adaptability to varying ore qualities.

2. Hydrometallurgy: Chemical Leaching for Limonite Ore
Hydrometallurgy is the star for limonite, using aqueous solutions to dissolve nickel and cobalt without extreme heat. The gold standard here is High-Pressure Acid Leaching (HPAL), a process works by grinding limonite into fine particles, mixing it with sulfuric acid, and subjecting the slurry to high temperature (240–260°C) and pressure in an autoclave. This dissolves nickel and cobalt into a “pregnant leach solution (PLS),” which is then purified via solvent extraction or ion exchange.
For smaller operations or lower-grade ores, heap leaching is a cost-effective alternative: ore is piled on impermeable pads, and dilute sulfuric acid is trickled over it to leach nickel. While simpler, it’s slower (recovery takes months) and less efficient.
In practice, this nickel extraction process requires precise control of slurry density, temperature, and agitation. This is where equipment selection makes a real difference. In our experience at Xinhai, using advanced agitation tanks and corrosion-resistant leaching equipment can significantly improve leaching efficiency while extending equipment life.
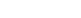
3. Comparing the Main Methods: Pyrometallurgy vs. Hydrometallurgy
Both pyrometallurgy and hydrometallurgy are proven, but they shine in different scenarios. Pyrometallurgy excels with saprolite ores, producing ferronickel efficiently but consuming significant energy through high-temperature smelting. It’s straightforward and scalable, as seen in many Indonesian smelters churning out material for steel mills. Hydrometallurgy, suited to limonite, operates at lower temperatures, extracting nickel and cobalt more selectively with less waste slag. However, it requires careful management of acidic solutions and autoclaves. Emerging options like atmospheric leaching or bioleaching offer greener alternatives but are less mature for large-scale use.
Here’s a quick comparison table based on typical industry data:
Aspect | Pyrometallurgy (RKEF) | Hydrometallurgy (HPAL) |
Suitable Ore Type | Saprolite (high MgO, Ni 1.6-2.5%) | Limonite (high Fe, Ni 0.8-1.5%, high Co) |
Nickel Recovery Rate | 92-97% | >90% (Ni and Co) |
Energy Consumption | High (400-500 kWh/t ore) | Lower (operates at lower temps) |
Capital/Operating Cost | Moderate to high for ferronickel | $8,000-10,000/mt Ni (for MHP) |
Environmental Impact | Higher CO₂ emissions, slag volume | Lower emissions but acid waste risks |
End Product | Ferronickel (for steel) | Nickel sulfate/MHP (for batteries) |
Conclusion
Every nickel laterite ore body is different, and copying a generic flowsheet often leads to disappointing results. Xinhai Mining approaches nickel laterite ore processing from a problem-solving perspective. Through detailed ore characterization, process testing, and customized equipment selection, we help clients overcome issues like low recovery, excessive acid consumption, or unstable operation.
Ready to boost your nickel processing efficiency? Contact Xinhai Mining today for professional consultation, customized process design, and relia


 marketing@ytxinhai.com
marketing@ytxinhai.com  0086 13810327080
0086 13810327080 
































































































 CHAT
CHAT MESSAGE
MESSAGE